VACCINE EXCIPIENT TABLE OF INGREDIENTS. WHY YOU SHOULD NOT GET VACCINATED WITH TOXINS
Vaccine Excipient Summary
As user of the Vaccines, you need to understand what the ingredients are, and what it can do to your physical system.
If it is a toxic pharmaceutical medical product or any other foreign substance or product it should be avoided at all costs.
Vaccine Excipient Summary
Excipients Included in U.S. Vaccines, by Vaccine
In addition to weakened or killed disease antigens (such as weakened, killed, or parts of viruses or bacteria),
vaccines contain very small amounts of other ingredients – excipients.
Some excipients are added to a vaccine for a specific purpose. These include:
y
Preservatives, to prevent contamination. For example, thimerosal.
y
Adjuvants, to help stimulate a stronger immune response. For example, aluminum salts.
y
Stabilizers, to keep the vaccine potent during transportation and storage. For example, sugars or gelatin.
Others are residual trace amounts of materials that were used during the manufacturing process and removed.
These can include:
y
Cell culture materials, used to grow the vaccine antigens. For example, egg protein, various culture media.
y
Inactivating ingredients, used to kill viruses or inactivate toxins. For example, formaldehyde.
y
Antibiotics, used to prevent contamination by bacteria. For example, neomycin.
The following table lists substances, other than active ingredients (i.e., antigens), shown in the manufacturers’
package insert (PI) as being contained in the final formulation of each vaccine. Substances used in the
manufacture of a vaccine but not listed as contained in the final product (e.g., culture media) can be found
in each PI, but are not shown on this table. Each PI, which can be found on the FDA’s website (see below)
contains a description of that vaccine’s manufacturing process, including the amount and purpose of each
substance. In most PIs, this information is found in Section 11: “Description.” Please refer to the PI for a complete
list of ingredients or excipients. A table listing vaccine excipients and media by excipient is published by the
Institute for Vaccine Safety at Johns Hopkins University, and can be found at http://www.vaccinesafety.edu/
components-Excipients.htm.
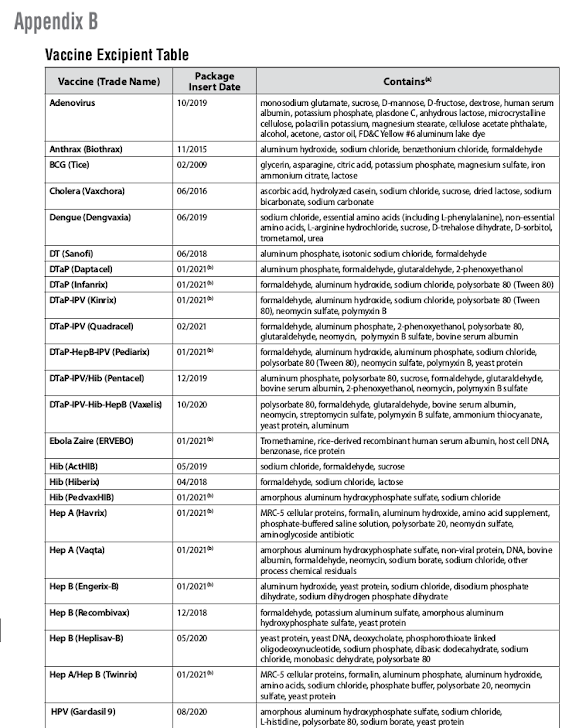
Appendix B
Vaccine Excipient Table
Vaccine (Trade Name)
B
Package
Insert Date
Contains (a)
Adenovirus 10/2019 monosodium glutamate, sucrose, D-mannose, D-fructose, dextrose, human serum
albumin, potassium phosphate, plasdone C, anhydrous lactose, microcrystalline
cellulose, polacrilin potassium, magnesium stearate, cellulose acetate phthalate,
alcohol, acetone, castor oil, FD&C Yellow #6 aluminum lake dye
Anthrax (Biothrax) 11/2015 aluminum hydroxide, sodium chloride, benzethonium chloride, formaldehyde
BCG (Tice) 02/2009 glycerin, asparagine, citric acid, potassium phosphate, magnesium sulfate, iron
ammonium citrate, lactose
Cholera (Vaxchora) 06/2016 ascorbic acid, hydrolyzed casein, sodium chloride, sucrose, dried lactose, sodium
bicarbonate, sodium carbonate
Dengue (Dengvaxia) 06/2019 sodium chloride, essential amino acids (including L-phenylalanine), non-essential
amino acids, L-arginine hydrochloride, sucrose, D-trehalose dihydrate, D-sorbitol,
trometamol, urea
DT (Sanofi) 06/2018 aluminum phosphate, isotonic sodium chloride, formaldehyde
DTaP (Daptacel) 01/2021 aluminum phosphate, formaldehyde, glutaraldehyde, 2-phenoxyethanol
DTaP (Infanrix) 01/2021 formaldehyde, aluminum hydroxide, sodium chloride, polysorbate 80 (Tween 80)
DTaP-IPV (Kinrix) 01/2021 (b) formaldehyde, aluminum hydroxide, sodium chloride, polysorbate 80 (Tween
80), neomycin sulfate, polymyxin B
DTaP-IPV (Quadracel) 02/2021 formaldehyde, aluminum phosphate, 2-phenoxyethanol, polysorbate 80,
glutaraldehyde, neomycin, polymyxin B sulfate, bovine serum albumin
DTaP-HepB-IPV (Pediarix) 01/2021 (b) formaldehyde, aluminum hydroxide, aluminum phosphate, sodium chloride,
polysorbate 80 (Tween 80), neomycin sulfate, polymyxin B, yeast protein
DTaP-IPV/Hib (Pentacel) 12/2019 aluminum phosphate, polysorbate 80, sucrose, formaldehyde, glutaraldehyde,
bovine serum albumin, 2-phenoxyethanol, neomycin, polymyxin B sulfate
DTaP-IPV-Hib-HepB (Vaxelis) 10/2020 polysorbate 80, formaldehyde, glutaraldehyde, bovine serum albumin,
neomycin, streptomycin sulfate, polymyxin B sulfate, ammonium thiocyanate,
yeast protein, aluminum
Ebola Zaire (ERVEBO) 01/2021 (b) Tromethamine, rice-derived recombinant human serum albumin, host cell DNA,
benzonase, rice protein
Hib (ActHIB) 05/2019 sodium chloride, formaldehyde, sucrose
Hib (Hiberix) 04/2018 formaldehyde, sodium chloride, lactose
Hib (PedvaxHIB) 01/2021 (b) amorphous aluminum hydroxyphosphate sulfate, sodium chloride
Hep A (Havrix) 01/2021 MRC-5 cellular proteins, formalin, aluminum hydroxide, amino acid supplement,
phosphate-buffered saline solution, polysorbate 20, neomycin sulfate,
aminoglycoside antibiotic
Hep A (Vaqta) 01/2021 (b) amorphous aluminum hydroxyphosphate sulfate, non-viral protein, DNA, bovine
albumin, formaldehyde, neomycin, sodium borate, sodium chloride, other
process chemical residuals
Hep B (Engerix-B) 01/2021 (b) aluminum hydroxide, yeast protein, sodium chloride, disodium phosphate
dihydrate, sodium dihydrogen phosphate dihydrate
Hep B (Recombivax) 12/2018 formaldehyde, potassium aluminum sulfate, amorphous aluminum
hydroxyphosphate sulfate, yeast protein
Hep B (Heplisav-B) 05/2020 yeast protein, yeast DNA, deoxycholate, phosphorothioate linked
oligodeoxynucleotide, sodium phosphate, dibasic dodecahydrate, sodium
chloride, monobasic dehydrate, polysorbate 80
Hep A/Hep B (Twinrix) 01/2021 (b) MRC-5 cellular proteins, formalin, aluminum phosphate, aluminum hydroxide,
amino acids, sodium chloride, phosphate buffer, polysorbate 20, neomycin
sulfate, yeast protein
HPV (Gardasil 9) 08/2020 amorphous aluminum hydroxyphosphate sulfate, sodium chloride,
L-histidine, polysorbate 80, sodium borate, yeast protein
Appendix B-8
(b)
(b)
(b)Appendix B
Vaccine (Trade Name)
Package
Insert Date
Contains (a)
Influenza (Afluria)
Quadrivalent (c) 07/2020 sodium chloride, monobasic sodium phosphate, dibasic sodium phosphate,
monobasic potassium phosphate, potassium chloride, calcium chloride, sodium
taurodeoxycholate, ovalbumin, sucrose, neomycin sulfate, polymyxin B, beta-
propiolactone, hydrocortisone, thimerosal (multi-dose vials)
Influenza (Fluad) (c) 10/2020 squalene, polysorbate 80, sorbitan trioleate, sodium citrate dehydrate, citric
acid monohydrate, neomycin, kanamycin, hydrocortisone, egg proteins,
cetyltrimethylammonium bromide (CTAB), formaldehyde
Influenza (Fluad)
Quadrivalent (c) 11/2020 squalene, polysorbate 80, sorbitan trioleate, sodium citrate dihydrate, citric acid
monohydrate, neomycin, kanamycin, hydrocortisone, egg protein, formaldehyde
Influenza (Fluarix)
Quadrivalent (c) 07/2020 octoxynol-10 (TRITON X-100), α-tocopheryl hydrogen succinate, polysorbate
80 (Tween 80), hydrocortisone, gentamicin sulfate, ovalbumin, formaldehyde,
sodium deoxycholate, sodium phosphate-buffered isotonic sodium chloride
Influenza (Flublok)
Quadrivalent (c) 06/2020 sodium chloride, monobasic sodium phosphate, dibasic sodium phosphate,
polysorbate 20 (Tween 20), baculovirus and Spodoptera frugiperda cell proteins,
baculovirus and cellular DNA, Triton X-100
Influenza (Flucelvax)
Quadrivalent (c) 03/2020 Madin Darby Canine Kidney (MDCK) cell protein, phosphate buffered saline,
protein other than HA, MDCK cell DNA, polysorbate 80, cetyltrimethlyammonium
bromide, and β-propiolactone, thimerosal (multi-dose vials)
Influenza (Flulaval)
Quadrivalent (c) 2020 ovalbumin, formaldehyde, sodium deoxycholate, α-tocopheryl hydrogen
succinate, polysorbate 80, phosphate-buffered saline solution
Influenza (Fluzone)
Quadrivalent (c) 2020 formaldehyde, egg protein, octylphenol ethoxylate (Triton X-100), sodium
phosphate-buffered isotonic sodium chloride solution, thimerosal (multi-dose
vials)
Influenza (Fluzone)
High Dose (c) 2020 egg protein, octylphenol ethoxylate (Triton X-100), sodium phosphate-buffered
isotonic sodium chloride solution, formaldehyde
Influenza (FluMist)
Quadrivalent (c) 08/2020 monosodium glutamate, hydrolyzed porcine gelatin, arginine, sucrose, dibasic
potassium phosphate, monobasic potassium phosphate, ovalbumin, gentamicin
sulfate, ethylenediaminetetraacetic acid (EDTA)
IPV (Ipol) 01/2021 (b) calf bovine serum albumin, 2-phenoxyethanol, formaldehyde, neomycin,
streptomycin, polymyxin B, M-199 medium
Japanese Encephalitis (Ixiaro) 09/2018 aluminum hydroxide, protamine sulfate, formaldehyde, bovine serum albumin,
host cell DNA, sodium metabisulphite, host cell protein
MenACWY (Menactra) 04/2018 sodium phosphate buffered isotonic sodium chloride solution, formaldehyde,
diphtheria toxoid protein carrier
MenACWY (MenQuadfi) 01/2021 (b) sodium chloride, sodium acetate, formaldehyde
MenACWY (Menveo) 07/2020 formaldehyde, CRM 197 protein
MenB (Bexsero) 01/2021 aluminum hydroxide, sodium chloride, histidine, sucrose, kanamycin
MenB (Trumenba) 2018 polysorbate 80, aluminum phosphate, histidine buffered saline
MMR (MMR-II) 12/2020 sorbitol, sucrose, hydrolyzed gelatin, recombinant human albumin, neomycin,
fetal bovine serum, WI-38 human diploid lung fibroblasts
MMRV (ProQuad)
(Frozen: Recombinant
Albumin) 01/2021 (b) MRC-5 cells including DNA and protein, sucrose, hydrolyzed gelatin, sodium
chloride, sorbitol, monosodium L-glutamate, sodium phosphate dibasic,
recombinant human albumin, sodium bicarbonate, potassium phosphate
monobasic, potassium chloride, potassium phosphate dibasic, neomycin, bovine
calf serum, other buffer and media ingredients
(b)
PCV13 (Prevnar 13) 08/2017 CRM 197 carrier protein, polysorbate 80, succinate buffer, aluminum phosphate
PPSV-23 (Pneumovax) 09/2020 isotonic saline solution, phenol
Rabies (Imovax) 10/2019 human albumin, neomycin sulfate, phenol red, beta-propiolactone
Rabies (RabAvert) 2018 chicken protein, polygeline (processed bovine gelatin), human serum albumin,
potassium glutamate, sodium EDTA, ovalbumin, neomycin, chlortetracycline,
amphotericin B
Rotavirus (RotaTeq) 01/2021 (b) sucrose, sodium citrate, sodium phosphate monobasic monohydrate, sodium
hydroxide, polysorbate 80, cell culture media, fetal bovine serum
Appendix B-9
BAppendix B
Vaccine (Trade Name)
Package
Insert Date
Contains (a)
Rotavirus (Rotarix) 01/2021 (b) dextran, Dulbecco’s Modified Eagle Medium (sodium chloride, potassium
chloride, magnesium sulfate, ferric (III) nitrate, sodium phosphate, sodium
pyruvate, D-glucose, concentrated vitamin solution, L-cystine, L-tyrosine,
amino acids, L-glutamine, calcium chloride, sodium hydrogenocarbonate, and
phenol red), sorbitol, sucrose, calcium carbonate, sterile water, xanthan
[Porcine circovirus type 1 (PCV1) is present in Rotarix. PCV-1 is not known to
cause disease in humans.]
Smallpox
(Vaccinia) (ACAM2000) 03/2018 HEPES, 2% human serum albumin, 0.5 - 0.7% sodium chloride USP, 5% Mannitol
USP, neomycin, polymyxin B, 50% Glycerin USP, 0.25% phenol USP
Td (Tenivac) 11/2019 aluminum phosphate, formaldehyde, sodium chloride
Td (TDVAX) 09/2018 aluminum phosphate, formaldehyde, thimerosal
Tdap (Adacel) 12/2020 aluminum phosphate, formaldehyde, 2-phenoxyethanol, glutaraldehyde
Tdap (Boostrix) 09/2020 formaldehyde, aluminum hydroxide, sodium chloride, polysorbate 80
Typhoid (Typhim Vi) 03/2020 formaldehyde, phenol, polydimethylsiloxane, disodium phosphate, monosodium
phosphate, sodium chloride
Typhoid (Vivotif Ty21a) 9/2013 sucrose, ascorbic acid, amino acids, lactose, magnesium stearate, gelatin
Varicella (Varivax) Frozen 01/2021 (b) sucrose, hydrolyzed gelatin, sodium chloride, monosodium L-glutamate, sodium
phosphate dibasic, potassium phosphate monobasic, potassium chloride, MRC-5
human diploid cells including DNA & protein, sodium phosphate monobasic,
EDTA, neomycin, fetal bovine serum
Yellow Fever (YF-Vax) 2/2019 sorbitol, gelatin, sodium chloride
Zoster (Shingles) (Shingrix) 01/2021 (b) sucrose, sodium chloride, dioleoyl phosphatidylcholine (DOPC), 3-O-desacl-
4’monophosphoryl lipid A (MPL), QS-21 (a saponin purified from plant extract
Quillaja saponaria Molina), potassium dihydrogen phosphate, cholesterol,
sodium dihydrogen phosphate dihydrate, disodium phosphate anhydrous,
dipotassium phosphate, polysorbate 80, host cell protein and DNA
Abbreviations: DT = diphtheria and tetanus toxoids; DTaP = diphtheria and tetanus toxoids and acellular pertussis; Hep A = Hepatitis A; Hep B = Hepatitis B; Hib =
Haemophilus influenzae type b; HPV = human papillomavirus; IPV = inactivated poliovirus; LAIV = live, attenuated influenza vaccine; MenACWY = quadrivalent
meningococcal conjugate vaccine; MenB = serogroup B meningococcal vaccine; MMR = measles, mumps, and rubella; MMRV = measles, mumps, rubella, varicella; PCV13
= pneumococcal conjugate vaccine; PPSV23= pneumococcal polysaccharide vaccine; Td = tetanus and diphtheria toxoids; Tdap = tetanus toxoid, reduced diphtheria
toxoid, and acellular pertussis.
All information was extracted from manufacturers’ package inserts. The date shown in the Date column of the table is the edition date of the PI in use in January 2021 by
month and year. In some cases, only a year was printed on the PI. If in doubt about whether a PI has been updated since this table was prepared, check the FDA’s website
at:
(a)
http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm
The PI was not dated and this is the date the PI was reviewed for this table.
(b)
All influenza vaccine in this table are 2020-21 northern hemisphere formulation.
(c)
B
Appendix B-10
January 2021




Comments
Post a Comment